Aortic Regurgitation Trial Investigating
Surgery Versus Trilogy® Transcatheter
Heart Valve
Learn More
Study objective & design
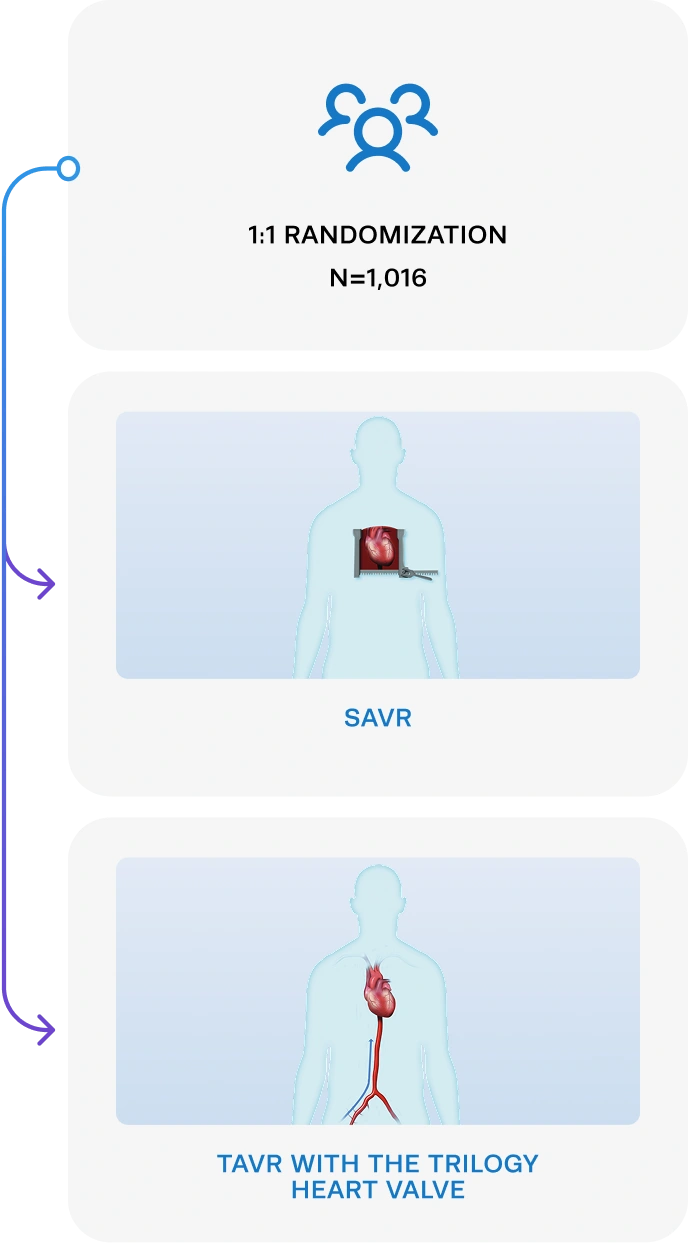
The ARTIST Trial is the world’s first study investigating TAVR versus SAVR in the AR population.
SAVR and TAVR with Trilogy
& 30 OUS Sites
Who should be considered?
Patients with ≥3+ native valve aortic regurgitation
requiring aortic valve replacement.



ARTIST trial site locator
Find a Participating ARTIST Trial Site Near You
Your patient may be eligible for an aortic regurgitation (AR) clinical trial.
Eligibility criteria
Eligibility Depends on Meeting All Inclusion Criteria
and None of the Exclusion Criteria.

-
Clinical indication for AVR for native valve predominant AR defined as:
- Class I or II indication for AVR according to ACC/AHA or ESC/EACTS guidelines with moderate to severe or severe AR (Grade 3+ or 4+) on transthoracic echocardiography, transesophageal echocardiography or cardiac MRI as assessed by the core laboratory
OR- AR severity that remains indeterminate despite core laboratory review of all imaging including at least one advanced imaging modality (TEE or cardiac MRI) AND evidence of left ventricular damage* from AR with unanimous agreement from the local heart team, an independent clinical evaluation committee and the CRB that the symptomatic subject (NYHA II or greater) will benefit from SAVR for AR.
- The subject is a candidate for TAVR using the Trilogy THV system and SAVR using a commercially approved bioprosthetic heart valve.
- Subject and the treating physician agree that the subject will return for all required post-procedure follow-up visits.
- Subject meets the legal minimum age to provide informed consent based on local regulatory requirements.
-
Reduced Left Ventricular (LV) Function:
- LV ejection fraction ≤55%1
- Decreased (≤17%) LV global longitudinal strain (GLS)2
-
LV Dilation or Damage:
- Left ventricular end systolic diameter (LVESD) >50mm1 or LVESD index (LVESDi) >20mm/m2,3
- Left ventricular end systolic volume index (LVESVi) >45 ml/m2,4,5
- Increase in LVEDD into the severe range (LVEDD >65 mm)1
- Progressive LV dilatation across a minimum of three sequential noninvasive studies separated by at least 2 months (baseline and 2 additional studies)
- The coexistence of LVEDV ≥246 ml and ≥33% AR regurgitant fraction (RF) assessed by CMR6,7
- Increased Cardiac Biomarkers Indicating LV Wall Stress: BNP ≥130 pg/ml or NT-proBNP ≥602 pg/ml (or NT-proBNP ratio ≥26.5)8

- Confirmed moderate (2+) or less AR severity by core laboratory evaluation
- Estimated life expectancy of less than 24 months due to associated non-cardiac comorbid conditions
- Subject is high-risk for SAVR as determined by the local heart team
- Subject refuses SAVR as a treatment option
- Subject refuses a blood transfusion
- Subject is selected for aortic valve repair or aortic surgery
- Marfan syndrome or other known connective tissue disease that would necessitate aortic root replacement/intervention
- Subject unable to undergo pre-procedure CT scan analysis for annular sizing
- Mitral or tricuspid disease, considered clinically significant, such that if randomized to surgery, repair, or replacement would be expected
- Subject has a known hypersensitivity or contraindication to all anticoagulation/antiplatelet medications (or inability to be anticoagulated for the index procedure), nitinol, or sensitivity to contrast media which cannot be adequately pre-medicated
- Hostile chest or conditions or complications from a prior surgery that would preclude safe reoperation (i.e., mediastinitis, radiation damage, chest wall abnormalities, adhesion of aorta or internal mammary artery (IMA) to the sternum)
- Need for emergency surgery or TAVR for any reason
- Cardiogenic shock manifested by low cardiac output, vasopressor dependence, or need for mechanical circulatory support
- Ongoing sepsis or active infective endocarditis with ongoing antibiotic (including suppressive) therapy or positive blood cultures within 6 weeks
- Cardiac resynchronization therapy (CRT) device implantation within 30 days of randomization
- LVEF <25% according to core laboratory measurement of resting echocardiogram at time of screening
- Moderate-to-severe or worse right ventricular dysfunction on resting echocardiogram according to core laboratory
- Severe chronic liver disease (Child-Pugh C) or any active liver disease
- Chronic Kidney Disease Stage 4 or 5 (<30 cc/min/1.73 m2 or dialysis)
- Severe Pulmonary Hypertension (pulmonary arterial systolic pressure >2/3 systemic systolic pressure)
- Severe Chronic Obstructive Pulmonary Disease (COPD) with FEV1 <50% predicted or need for chronic supplementary oxygen
- Blood dyscrasia as defined: leukopenia (WBC <3,000 Cells/μL), thrombocytopenia (platelet count <50,000 Cells/μL), or anemia (hemoglobin <9.0 g/dL) that is uncorrected prior to randomization
- History of bleeding diathesis or coagulopathy that is not adequately treated
- Active gastrointestinal bleeding precluding anticoagulation or antiplatelet therapy
- Any condition considered a contraindication to mechanical circulatory support
- Uncontrolled atrial fibrillation (i.e., resting heart rate >120 bpm)
- Evidence of an acute myocardial infarction ≤30 days before the index AVR
- Any percutaneous coronary or peripheral interventional procedure performed ≤30 days prior to AVR (subjects with placement of coronary or peripheral stent(s) should be assessed for the ability to safely proceed with SAVR within the protocol timeframe)
- Symptomatic carotid or vertebral artery disease or successful treatment of carotid stenosis within six weeks of randomization
- Prior stroke with residual modified Rankin Score ≥2
- Stroke or transient ischemic attack (TIA) within 6 months of randomization
- Body mass index (BMI) <20 or >50 kg/m2
- Currently participating in a cardiovascular investigational drug or device trial and has not yet completed follow-up for the primary endpoint (excluding registries)
- Severe cognitive decline (resulting in either inability to provide informed consent for the trial/procedure, prevents independent lifestyle outside of a chronic care facility, or will fundamentally complicate rehabilitation from the procedure or compliance with follow-up visits)
- Other medical, social, or psychological conditions that in the opinion of the investigator preclude the subject from appropriate consent or adherence to the protocol required follow-up exams
- Pregnancy or intent to become pregnant prior to completion of all protocol follow-up requirements
-
Anatomical exclusion criteria (ANY of the following):
- Congenital bicuspid, unicuspid, or quadricuspid aortic valve verified by echocardiography or CCT core laboratory
- Native aortic annulus perimeter <66 mm or >90 mm per the core laboratory reading of baseline cardiac CT imaging
- Iliofemoral arteries with vessel characteristics unsuitable for sheath passage (e.g., calcification, tortuosity) as determined by the case review board (CRB)
- Significant abdominal or thoracic aortic disease (such as porcelain aorta, aneurysm, severe calcification, aortic coarctation) that would preclude safe passage of the delivery system or cannulation and aortotomy for SAVR
- According to the CRB, a combination of aortic root angulation (angle between the plane of the aortic valve annulus and horizontal plane/vertebrae), sinus size, and straight length of the aorta that will not allow safe device delivery and THV deployment
- Sinus of Valsalva anatomy that would prevent adequate coronary perfusion after valve implantation
- According to core laboratory evaluation, severe aortic stenosis
- Uncorrected hypertrophic obstructive cardiomyopathy
- Echocardiographic or Multi-slice CT (MSCT) evidence of untreated intracardiac mass or vegetation
- Left ventricular thrombus
- Left atrial thrombus without continuous appropriate anticoagulation within 90 days of the study procedure
-
Complex coronary artery disease:
- Unprotected left main coronary artery disease ≥50%
- Syntax score >32 (in the absence of prior revascularization)
- Heart Team determines that optimal revascularization cannot be adequately performed with either CABG at the time of SAVR or PCI at least 30 days prior to THV implant
ARTIST trial site locator
Find a Participating ARTIST Trial Site Near You
Your patient may be eligible for an aortic regurgitation (AR) clinical trial.
Find a SitePotential risks
Adverse events that may be anticipated in this clinical study are believed to be consistent with those associated with other minimally invasive surgical and catheter-based procedures, including TAVR procedures.
Potential risks include but are not limited to the following: Vascular damage, vascular access complications, peripheral nerve injury and/or ischemia, cardiovascular injury, hypotension, hypertension, cardiogenic tamponade or pericardial effusion, arrhythmias and conductions system disorders which may require permanent pacemaker implantation, heart murmur, hemodynamic compromise or cardiogenic shock, heart failure or low cardiac output, cardiac arrest, angina pectoris, myocardial infarction, thrombus formation, embolization (e.g., air, calcification material, thrombus), cerebrovascular event (e.g., TIA, stroke, neurologic changes), pulmonary embolism, pulmonary edema, pleural effusion, respiratory compromise or respiratory failure, renal compromise or renal failure, allergic reaction/hypersensitivity to contrast media, medication, or device materials, inflammation, infection (e.g., endocarditis, access site infection) and sepsis, fever, pneumonia, hemorrhage or bleeding, possibly requiring intervention or transfusion, retroperitoneal bleeding, restenosis, syncope, anemia, abnormal laboratory values (e.g., electrolyte imbalance), exercise intolerance or weakness, paralysis, permanent disability, or other comorbid condition (new onset or worsening) or death.
References
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143(5): e72-e227.
- deCampos D, Teixeira R, Saleiro C, Botelho A, Goncalve L. Global longitudinal strain in chronic asymptomatic aortic regurgitation: systematic review. Echo Res Pract 2020; 7(3): 39-48.
- Desai MY, Svensson LG. Chronic Severe Aortic Regurgitation: Should We Lower Operating Thresholds? Circulation 2019; 140(13): 1045-7.
- Anand V, Yang L, Luis SA, et al. Association of Left Ventricular Volume in Predicting Clinical Outcomes in Patients with Aortic Regurgitation. J Am Soc Echocardiogr 2021; 34(4): 352-9.
- Yang LT, Anand V, Zambito EI, et al. Association of Echocardiographic Left Ventricular End-Systolic Volume and Volume-Derived Ejection Fraction With Outcome in Asymptomatic Chronic Aortic Regurgitation. JAMA cardiology 2021; 6(2): 189-98.
- Senapati A, Malahfji M, Debs D, et al. Regional Replacement and Diffuse Interstitial Fibrosis in Aortic Regurgitation: Prognostic Implications From Cardiac Magnetic Resonance. JACC Cardiovasc Imaging 2021; 14(11): 2170-82.
- Myerson SG, d'Arcy J, Mohiaddin R, et al. Aortic regurgitation quantification using cardiovascular magnetic resonance: association with clinical outcome. Circulation 2012; 126(12): 1452-60.
- Pizarro R, Bazzino OO, Oberti PF, et al. Prospective validation of the prognostic usefulness of B-type natriuretic peptide in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol 2011; 58(16): 1705-14.