The Trilogy System
The First FDA-Approved
Treatment for Severe Aortic
Regurgitation

So is our valve.
Introduction
A transcatheter solution for aortic regurgitation has been impossible—until now
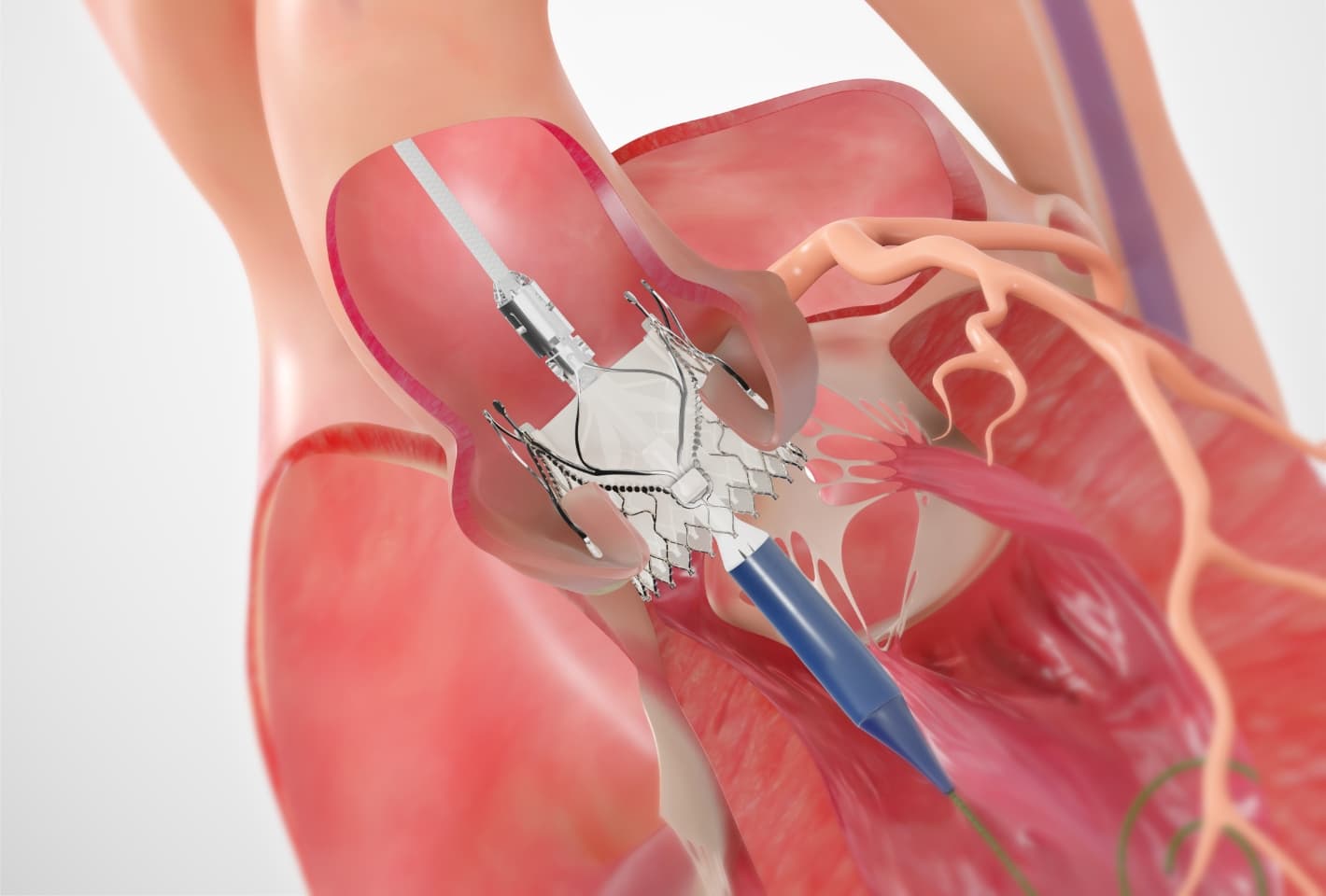
Other transcatheter heart valves are designed for aortic stenosis and rely on calcification in and around the annulus for anchoring and stability. In patients with aortic regurgitation, there is often no calcification, which means the valve is not able to anchor in place. The Trilogy valve is the only valve specifically designed for patients with aortic regurgitation.
The Trilogy Valve
future coronary access
with Jasmine® proprietary
tissue treatment
within the native cusps and
resist ventricular migration
commissural alignment
with native anatomy
the left ventricular outflow tract (LVOT)

Features
- Large, open cell design enables future coronary access
- Porcine pericardial tissue, with Jasmine® proprietary tissue treatment
- Self-expanding nitinol frame
- Locator technology allows commissural alignment with native anatomy
- Sealing ring provides anchoring and conforms to the left ventricular outflow tract (LVOT)
- Locators are positioned within the native cusps and resist ventricular migration
The Trilogy Delivery System
The delivery system has been engineered specifically for precise and reliable single-action valve deployment within the patient anatomy. It utilizes a catheter deflection mechanism to center the valve above the annulus and integrated rotation and axial positioning for simple and straightforward commissural alignment.
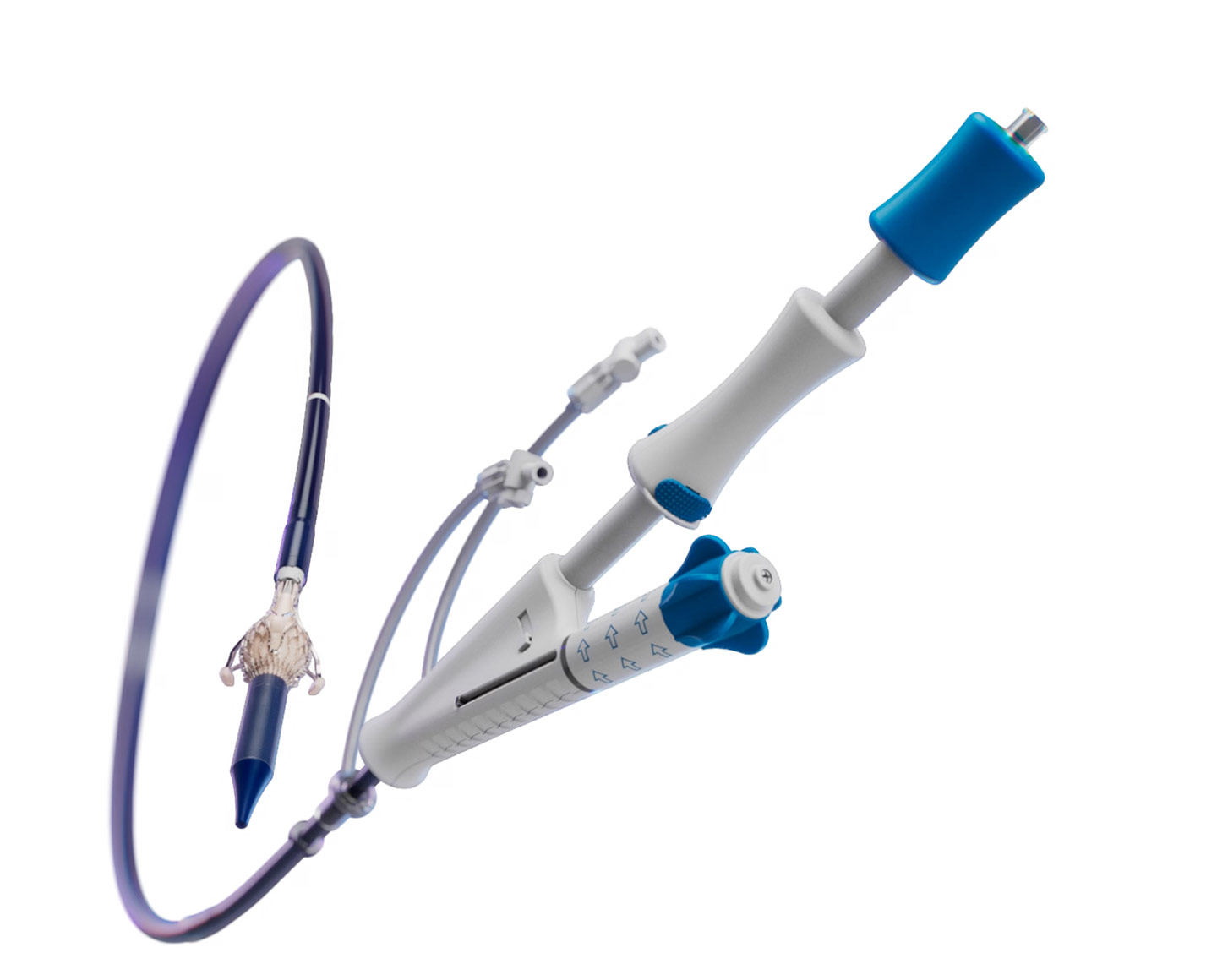
Features
- Single action deployment through a simple release mechanism
- Responsive rotation allows for straightforward commissural alignment
- Catheter deflection allows centralizing of Trilogy Valve above the annulus
through a simple
release mechanism
allows for straightforward
commissural alignment
allows centralizing of
Trilogy Valve above the
annulus

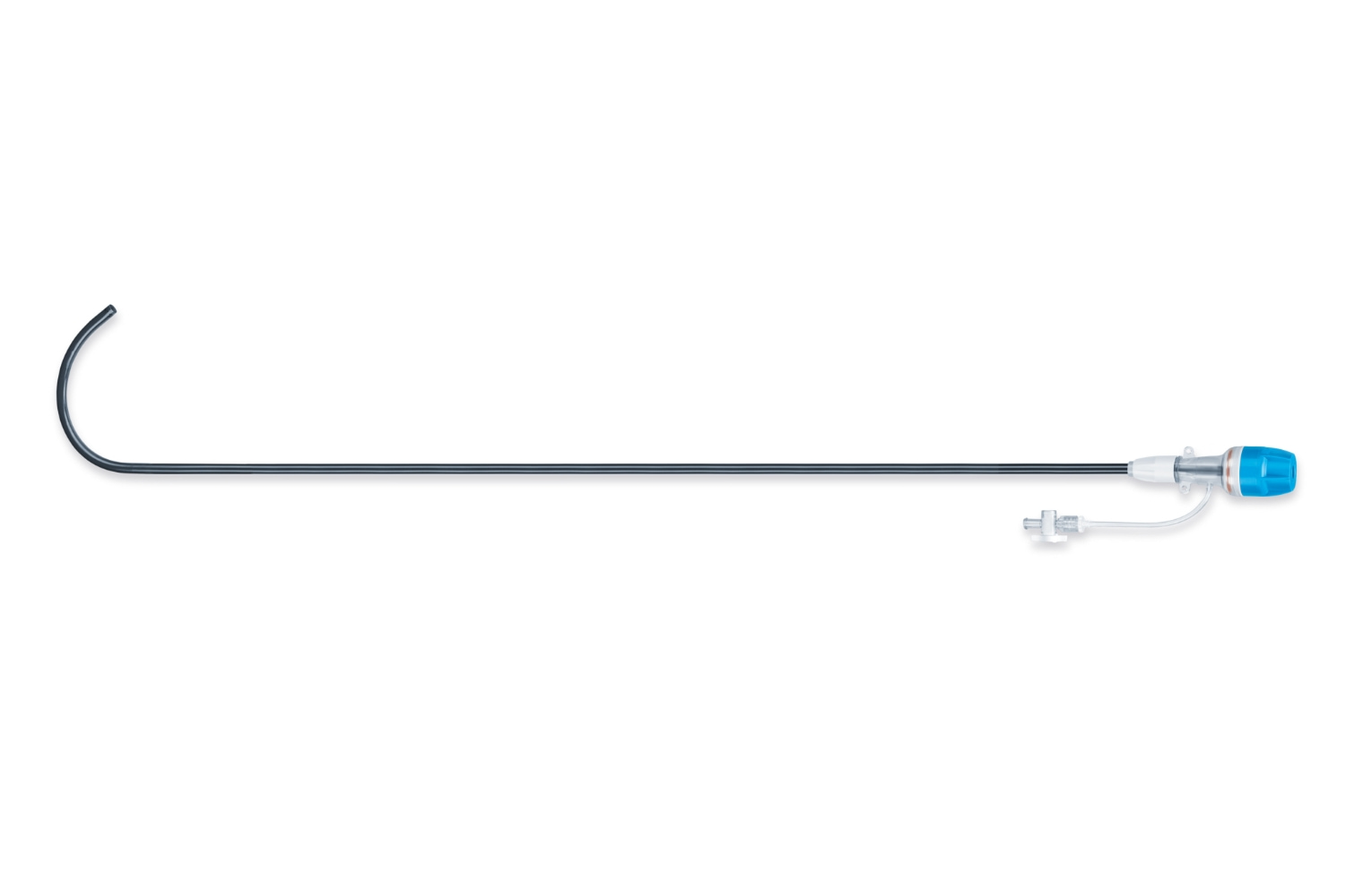
The Trilogy Introducer Sheath
Unlike other sheaths that only reach the abdominal aorta, the 85 cm pre-shaped, hydrophilically coated Trilogy Sheath extends to the sinotubuluar junction. This ensures the Trilogy Valve and your patient are protected all the way until release.

Features
- The Trilogy sheath facilitates safe vascular access and deliverability of the Trilogy Delivery System
- 85cm working length pre-shaped sheath that provides protection all the way to the ascending aorta, down to the sinotubular junction
vascular access and deliverability
of the Trilogy Delivery System
sheath that provides protection all
the way to the ascending aorta,
down to the sinotubular junction
See the Trilogy System in action
AR is different.
So is our valve.
In 700 patients with aortic regurgitation**
30-day outcomes in real-world patients
Trace PVL
(n=476)
Improvements in NYHA
functional class
At 30 days, patients showed marked improvements in symptoms with 92% of patients in NYHA I/II (vs 61% in NYHA III/IV at baseline).
See how the Trilogy System
is designed to treat severe
AR patients
Learn more about the
causes, symptoms, and
treatment of AR

Get in touch today to learn more about JenaValve and the Trilogy System
*Indications: The Trilogy® Transcatheter Heart Valve System is indicated for use in patients with native symptomatic, severe trileaflet aortic regurgitation (AR not due to acute endocarditis, rheumatic heart disease, or acute aortic dissection) who are judged by a Heart Team (including a cardiac surgeon), to be at high or greater risk for surgical aortic valve replacement (AVR), with an STS score ≥ 8% at 30 days, or other comorbidities (e.g., porcelain aorta, frailty, chest wall irradiation) that are not captured by the STS risk calculator.
Contraindications: The JenaValve Trilogy Transcatheter Heart Valve System is contraindicated for use in patients who have known hypersensitivity or contraindication to aspirin, heparin, ticlopidine or clopidogrel, nitinol alloy (nickel and titanium), tantalum or allergy to contrast agents that cannot be managed with premedication, or who have active bacterial endocarditis or other active infection.
CAUTION: Federal (United States) law restricts these devices to sale by or on the order of a physician.
**Moderate-severe/severe
†n=476
1Tamm, A. Jenavalve Trilogy TAVR System for the Treatment of Aortic Regurgitation Real-World Results from a Multicenter Cohort. Presented at TCT 2022, Sept. 18, 2022, Boston, MA.




