The only
TAVR system
FDA-approved
for aortic
regurgitation.

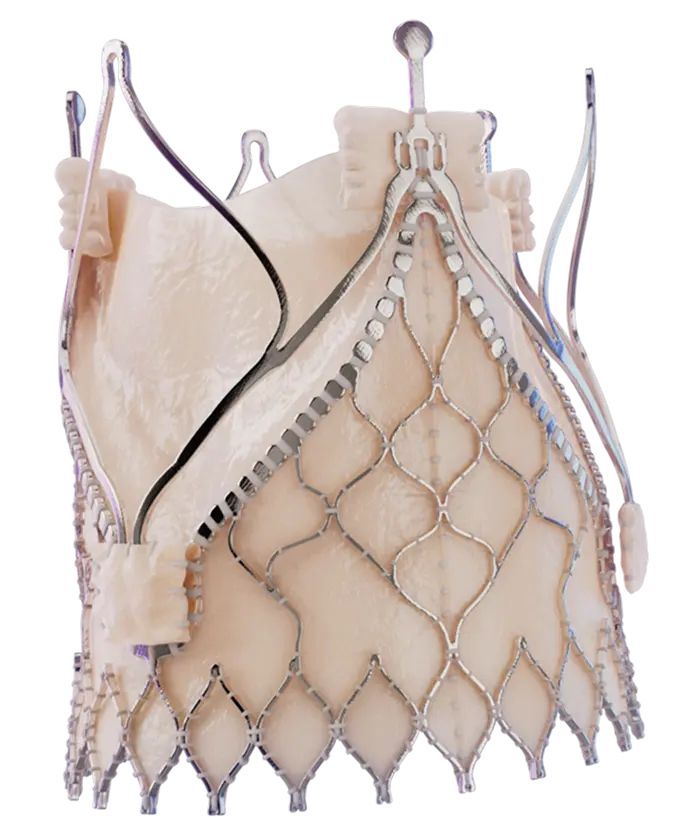
INTRODUCING
Trilogy
Transcatheter
Heart Valve

The valve that
makes TAVR for
AR possible

The Trilogy Valve with locator technology
You can finally give high-risk patients with severe, symptomatic aortic regurgitation (AR) an FDA-approved transcatheter aortic valve replacement (TAVR) treatment option. Trilogy's locator technology ensures commissural alignment and allows the valve to attach to the native leaflets for secure anchoring, even in the absence of calcium.



The Trilogy Valve with locator technology
You can finally give high-risk patients with severe, symptomatic aortic regurgitation (AR) an FDA-approved transcatheter aortic valve replacement (TAVR) treatment option. Trilogy’s locator technology ensures commissural alignment and allows the valve to attach to the native leaflets for secure anchoring, even in the absence of calcium.


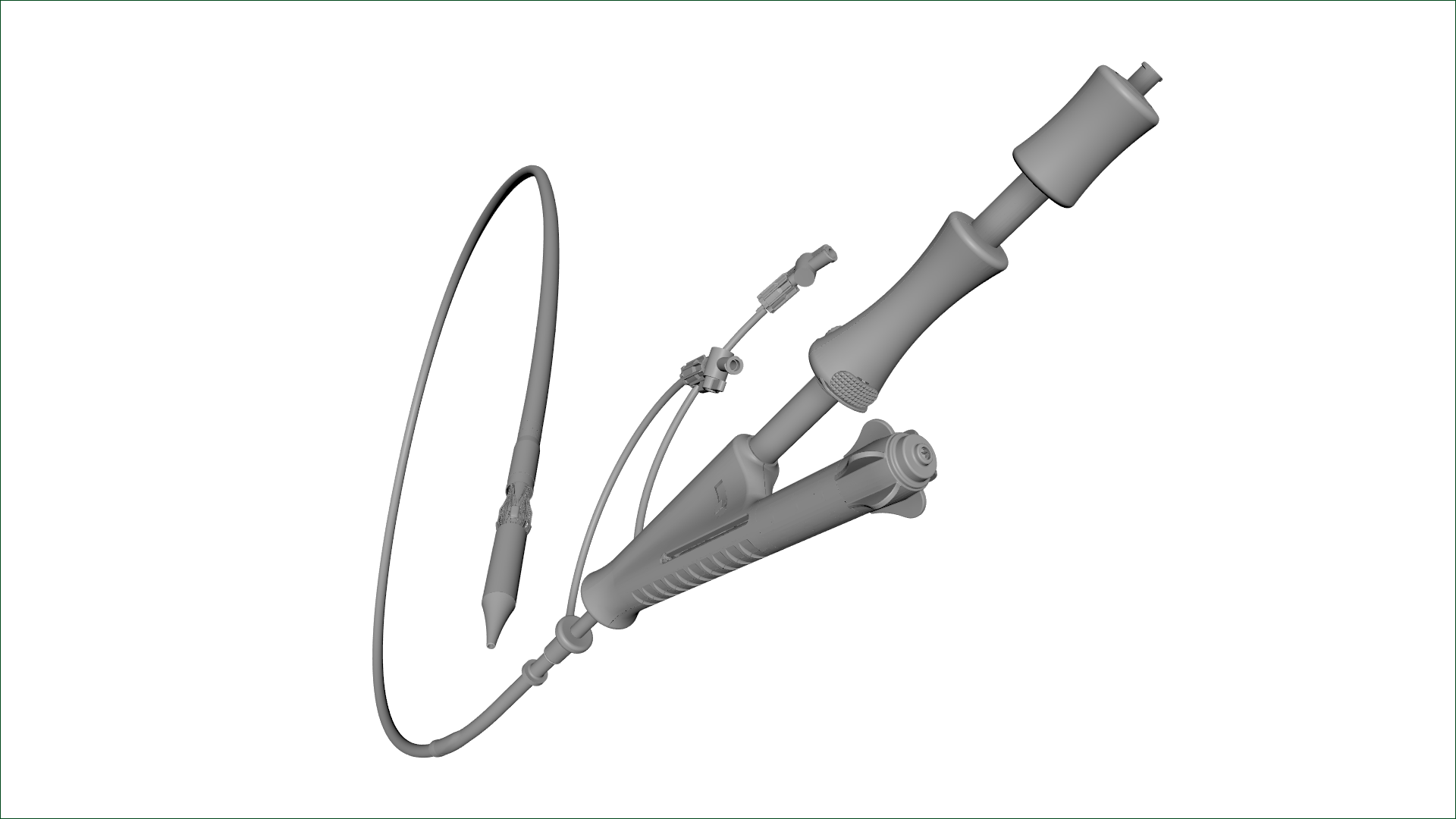
The Trilogy Delivery System
The delivery system has been engineered specifically for precise and reliable single-action valve deployment within the patient anatomy. It utilizes a catheter deflection mechanism to center the valve above the annulus and integrated rotation and axial positioning for simple and straightforward commissural alignment.


The Trilogy Introducer Sheath
Unlike other sheaths that only reach the abdominal aorta, the 85 cm pre-shaped Trilogy sheath extends into the ascending aorta. This ensures that the valve, delivery system, and your patient are protected as you navigate over the arch, all the way until the valve alignment and positioning.

TAVR Procedure
See Trilogy System in action
A transcatheter solution for aortic regurgitation has been impossible—until now.
(n=180)
(n=180)
(n=141)
(n=700)
(n=700)
(n=360)
Explore Clinical Evidence From the ALIGN-AR Study
See how the TrilogyTM Heart Valve System performed in the ALIGN-AR study—the first and only FDA-approved TAVR for AR.


Download device brochure
See how the Trilogy System is designed to treat severe AR patients
Download Brochure

*Indications:
The JenaValve Trilogy Heart Valve System is indicated for use in patients with native symptomatic, severe aortic regurgitation (AR) or symptomatic, severe aortic stenosis (AS), who are judged by a Heart Team (including a cardiac surgeon), to be at high or greater risk for surgical aortic valve replacement (AVR), with an STS score ≥ 8% at 30 days, or other comorbidities (e.g., porcelain aorta, frailty, chest wall irradiation) that are not captured by the STS risk calculator.
†n=49
1. Tamm, A. Jenavalve Trilogy TAVR System for the Treatment of Aortic Regurgitation Real-World Results from a Multicenter Cohort. Presented at TCT 2022, Sept. 18, 2022, Boston, MA.

 Scroll to explore
Scroll to explore 

 Menu
Menu  Close
Close